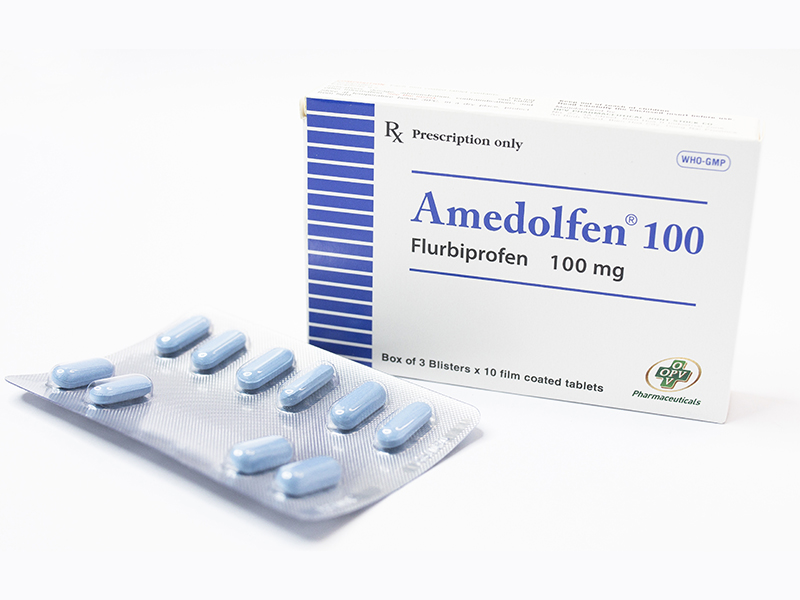
- Drug name: Amedofen
- Active ingredient name, Concentration – Content: Flurbiprofen 100mg
- Specification, Dosage form, Route of administration: Box of 3 blisters x 10 Tablets, Oral
- Expiry date (month): 24 months
- Registration number or license: VD-29055-18
- Manufacturing facility – manufacturing country: OPV Pharmaceutical Joint Stock Company – Vietnam


Gửi bình luận