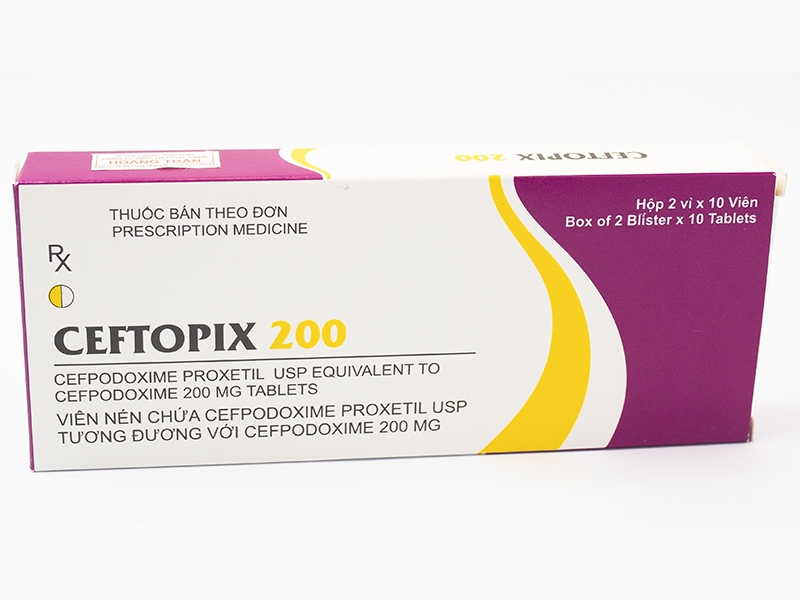
- Drug name: Ceftopix 200
- Active ingredient name, Concentration – Content: Cefpodoxime 200 mg
- Specification, Dosage form, Route of administration: Box of 2 blisters*10 tablets, Pills, Oral
- Expiry date (month): 24 months
- Registration number or license: VN-17289-13
- Manufacturing facility – manufacturing country: Cadila Pharmaceuticals Pvt., Ltd – India


Gửi bình luận