By: baoanhgroup
-
Tháng Mười 25.2023
-
Products And Activities
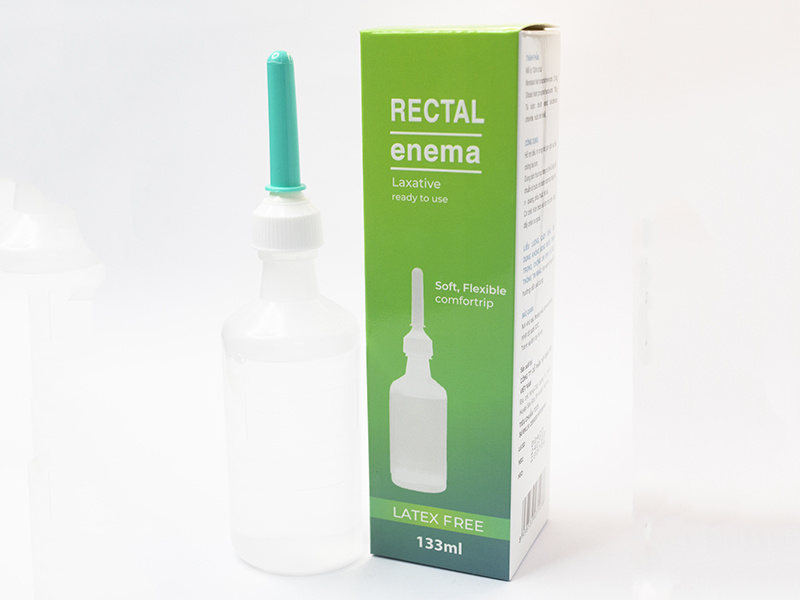
- Drug name: Laxafred
- Active ingredient name, Concentration – Content: Monobasic sodium phosphate monohydrate 21.41g; Dibasic sodium phosphate heptahydrate 7.89g; Excipients: disodium edetate, benzalkonium chloride, purified water.
- Specification, Dosage form, Route of administration: Box of 1 vial of 133ml, rectal enema
- Expiry date (month): 36 months
- Registration number or license: 220003019/PCBB-HN
- Manufacturing facility – manufacturing country: Vietnam TGD Group Joint Stock Company/ Vietnam


Gửi bình luận